What is new in pharma compliance tech?
Dual compliance, Continuous Aircraft Mapping and Monitoring, and more
Join Eupry's Q1 2026 product launch webinar to get an update on the latest technology in modern GxP compliance.
When: March 31st, 2026
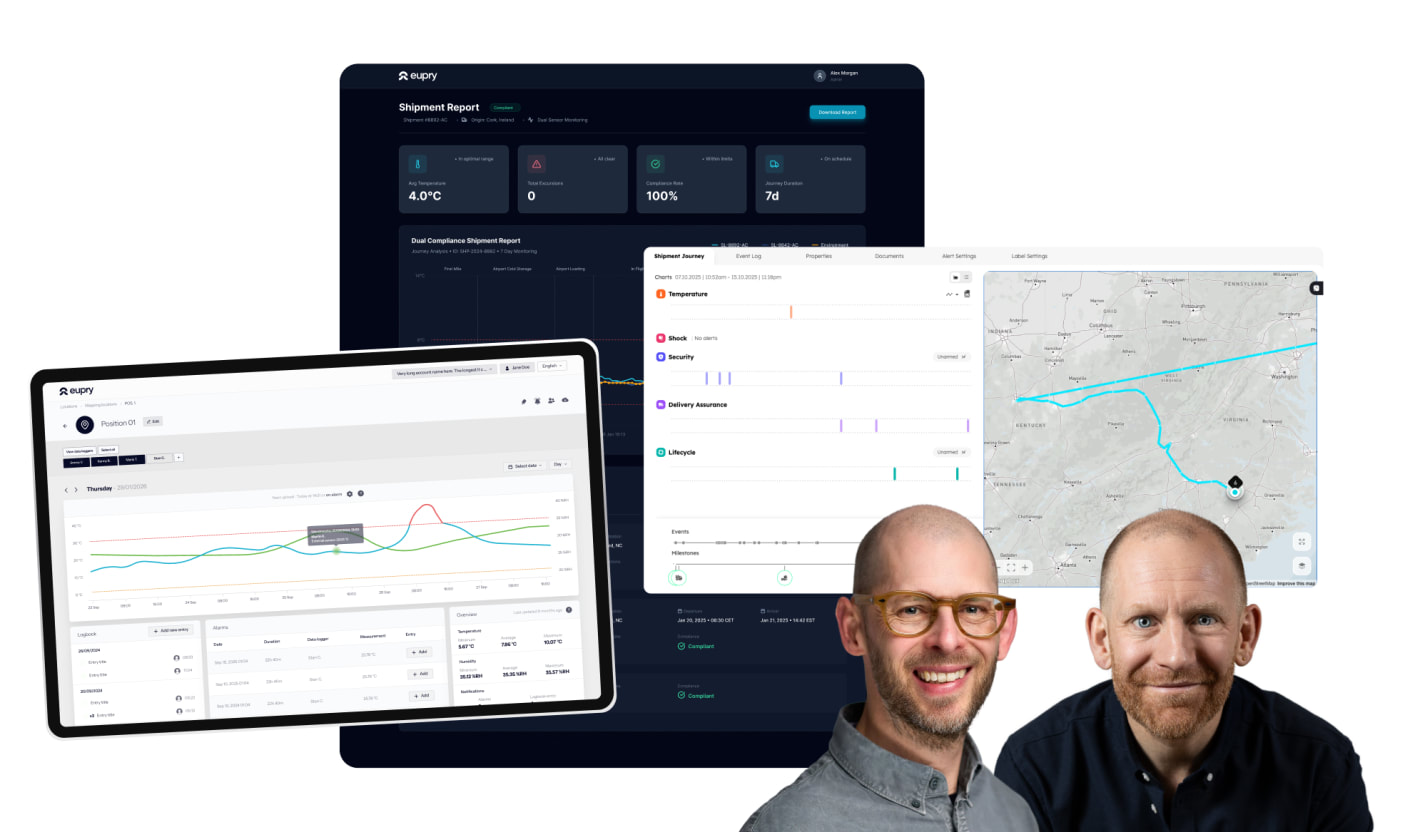
Temperature compliance should not mean endless manual checks, fragmented systems, or scrambling before audits. But for most pharma and logistics teams, that is still the reality.
Our Q1 releases tackle exactly that – centralized compliance control, automated workflows, and confidence that no issues go unnoticed.
Join us for a live walkthrough of what is now possible, what it means for your operation, and how to get the most out of these new capabilities.
Sign up now
Whether you are using Eupry, evaluating new solutions, or simply curious about what is new in GxP compliance technology, Eupry's quarterly product launches are for you.
- 9.30 AM CDT
- 10.30 AM EDT
- 3.30 P.M BST
- 4.30 PM CST
Key takeaways
- How status dashboards can be used to gain full control over compliance across all sites
- What dual compliance is and why it is relevant for reducing shipment monitoring costs
- How continuous mapping and monitoring for aircrafts* works (and will close the biggest cold chain blind spot)
- Best practices and real examples of utilizing the new tecnology + what is next in GxP compliance tech
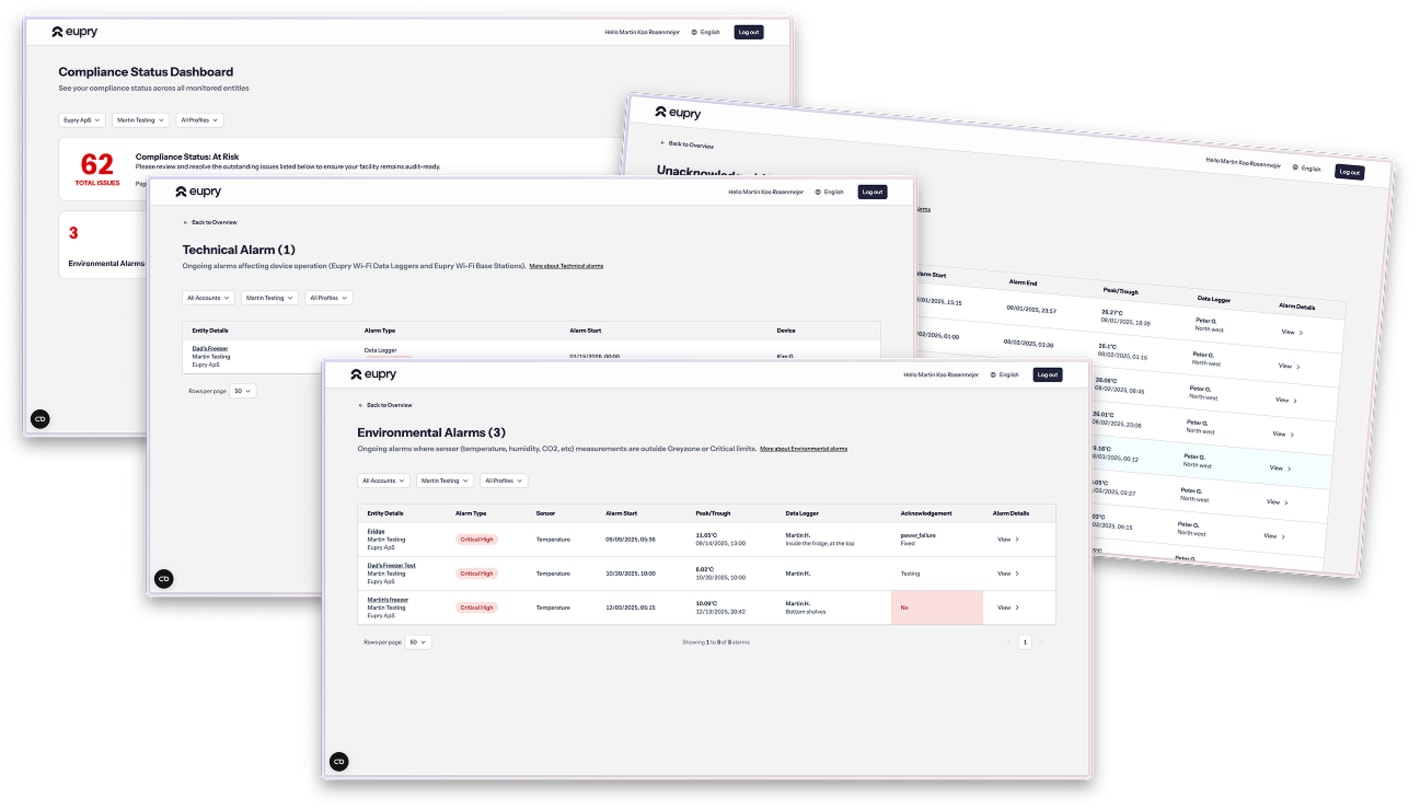
What you will see in the webinar
- Compliance Status Dashboard: All sites, all alarms, one view – full control across the entire organization.
- Dual Compliance cold chain platform: The first solution connecting facility and shipment data into one unified platform.
- Mapping software updates: Improvements to our GxP mapping data platform. Analyze distribution, identify hot/cold spots, and generate digital reports.
- Continuous aircraft mapping and monitoring: The world's first permanent CMM system for pharmaceutical air freight.

About Eupry
Eupry is a leader in thermal compliance technology, offering a suite of services and solutions keeping pharmaceutical, healthcare logistics, and other enterprises working in the GxP space compliant.
The speakers
- Martin Koo Rosenmejer, VP of Product at Eupry: Martin has almost 15 years of experience developing tech products in regulated and complex industries. Today, he leads product development at Eupry and is responsible for developing technology that simplifies compliance operations while meeting the standards pharmaceutical operations require.
- Anders Buchmann, VP of Commercial at Eupry: Anders works closely with pharma and logistics companies worldwide to optimize validation and compliance processes. He brings real-world insight from these organizations, understanding the operational challenges of GxP compliance.
